



India's pharmaceutical industry, known as the 'pharmacy of the world', has transformed affordable generic medicines into a global public good. By leveraging unique intellectual property laws and cost-effective manufacturing, India has broken monopolies on life-saving drugs like HIV/AIDS and COVID-19, redefining global health equity.
Copyright infringement not intended
Picture Courtesy: THE HINDU
India's pharmaceutical sector, known as the "Pharmacy of the World," faces challenges in bilateral trade agreement negotiations with the US due to tariffs. To strengthen its negotiating power and address global health needs, India must strategically position its generics as a global public good.
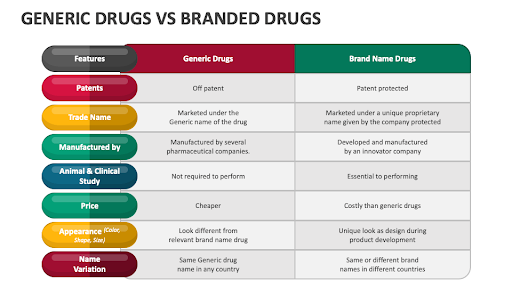
It is a pharmaceutical product that contains the same active ingredient, strength, safety, quality, and intended use as its branded counterpart.
Generic medicines, unlike branded drugs, can be manufactured and sold by multiple companies after the original patent expires, making them more affordable due to lower research, development, and marketing costs.
The Central Drugs Standard Control Organisation (CDSCO), under the Ministry of Health and Family Welfare, regulates and approves all drugs in India, including generics.
|
Regulation of Indian Pharmaceutical Industry Drugs and Cosmetics Act, 1940: Sets rules for making, selling, and testing drugs to ensure quality. Central Drugs Standard Control Organisation (CDSCO): Led by the Drugs Controller General of India (DCGI), it approves new drugs, oversees clinical trials, and ensures generics are safe. National Pharmaceutical Pricing Authority (NPPA): Caps prices of essential medicines to keep them affordable. Patents Act, 1970: Protects new inventions but blocks “evergreening” (extending patents with minor tweaks) to allow cheaper generic drugs after patents expire. Medical Devices Rules, 2017: Regulate medical devices, establishing licensing and quality control requirements for manufacturers and importers. New Drugs and Clinical Trials Rules, 2019: Provisions for compensating participants for trial-related harm and require registration of clinical research organizations. National Pharmaceutical Policy 2023: Align with international standards
|
India dominates the global generic medicine market, supplying affordable drugs to over 200 countries.
Global Leader in Generics: India accounts for 20% of global generic drug exports by volume, providing 40% of generics used in the U.S. and 50% in Africa. (PIB)
Vaccine Powerhouse: India supplies 60% of global vaccines, including those for HIV, tuberculosis, and malaria, supporting programs by WHO and UNICEF.
Export Growth: India’s pharmaceutical exports reached $27.82 billion in FY24, with the U.S. accounting for 31.35% of the total.
India’s generics are vital for affordable healthcare, particularly in the Global South, making them a critical global public good.
|
Initiatives taken by Government to Promote Quality Generic Medicine in India Pradhan Mantri Bhartiya Janaushadhi Pariyojana (PMBJP): Expanded network of over 16,000 Jan Aushadhi Kendras offering quality generics at 50-90% lower prices. Over a decade it saved consumers an estimated ₹30,000 crore. Mandatory generic prescriptions: National Medical Commission mandates doctors to prescribe drugs using generic names to encourage rational and affordable prescribing. Quality control: Generic medicines sold through PMBJP are sourced from WHO-GMP (Good Manufacturing Practice) standards certified suppliers. Price controls: The NPPA sets ceiling prices for essential medicines under the Drugs (Prices Control) Order, making them affordable for the public. National Health Mission (NHM): The NHM supports free provision of essential generic drugs in public health facilities to reduce out-of-pocket healthcare expenses for citizens. State-level programs: Many state governments (eg. Delhi) run their own free drug distribution programs in public hospitals, mandating the use of generics to ensure affordability. |
Promote Generics as Global Public Good
Emphasize India’s role in supplying affordable medicines to the Global South and developed nations. For example, Indian generics constitute 90% of U.S. prescriptions for diabetes, anxiety, depression, and cancer. (The Hindu)
Advocate TRIPS Flexibilities
Push for a comprehensive review of the TRIPS Agreement to protect public health, maintaining India’s stance on compulsory licensing and the Patents Act.
Propose Joint Ventures
Encourage joint ventures with U.S. and Global South pharmaceutical industries to promote technology transfer and collaborative R&D, enhancing India’s bargaining power.
Diversify Markets
Expand exports to West Asia, Central Asia, Africa, and South America, where demand for generics is growing.
Quality Control Failures
In 2022, Indian-made cough syrups caused child deaths in Gambia due to contamination. The US FDA flagged Indian firms for data faking and poor factory conditions.
API Dependency
India imports 70-80% of Active Pharmaceutical Ingredients (APIs) from China, creating supply chain vulnerabilities.
Trade Protectionism
U.S. tariffs and potential EU restrictions threaten India’s export growth.
Fragmented Regulatory System
The Central Drugs Standard Control Organisation (CDSCO) sets standards, but State Drug Regulatory Authorities (SDRAs) handle licensing and inspections, leading to uneven enforcement.
Struggling with Global Standards
Large companies meet US FDA and EU standards, but smaller firms lack funds for advanced equipment or training, limiting their exports.
Bioequivalence concerns
While generic drugs contain the same active ingredients, differences in inactive ingredients (excipients) and manufacturing processes can impact a drug's performance, stability, and absorption.
Limited R&D and Innovation
Indian firms spend less on R&D, focusing on generics over new drugs like biologics.
Lack of trained experts in biotechnology and regulatory affairs slows innovation.
Intellectual Property Rights (IPR) Issues
India’s laws block “evergreening” to favor generics, resulting legal fights with multinational firms. The US has raised concerns over this policy.
Weak protection of research data risks innovation losses. Many foreign firms avoid local manufacturing, limiting India’s tech gains.
Centralize drug regulation
Establish a single, transparent, and independent authority to ensure consistent quality standards and enforcement nationwide, addressing the current fragmented system.
Enhance Regulatory Compliance
Strengthen the Central Drugs Standard Control Organization (CDSCO) and align with WHO GMP (Good Manufacturing Practices) standards to reduce U.S. and EU rejection.
Upgrade testing
Modernize drug-testing labs and use technologies like blockchain and AI for end-to-end supply chain transparency, quality control, and fraud prevention.
Standardize bioequivalence
Require mandatory bioequivalence (BE) testing for all generic categories, harmonizing with international standards to boost reliability and consumer confidence.
Boost Domestic API Production
Expand the Production-Linked Incentive (PLI) scheme for API manufacturing, to reduce reliance on China.
Increase R&D Investment
Offer tax incentives for R&D, to develop high-value products like biologics and biosimilars through targeted incentives.
Adopt Sustainable Practices
Implement zero-waste technologies and renewable energy in manufacturing to meet global sustainability standards.
Promote Public-Private Partnerships
Collaborate with academia and global firms to advance biotechnology and digital health, supporting initiatives like the India-US TRUST framework.
Expand Jan Aushadhi Scheme
Scale up the Pradhan Mantri Bhartiya Janaushadhi Pariyojana Kendras, to ensure affordable generics in other countries, backing India’s global image. Eg. India’s first overseas Jan Aushadi Kendra inaugurated in Mauritius.
The Indian pharmaceutical sector must strategically position its generics as a global public good in trade negotiations with the U.S. By advocating TRIPS flexibilities, proposing joint ventures, and diversifying markets, India can counter U.S. tariffs and IP pressures while promoting public health.
Source: THE HINDU
|
PRACTICE QUESTION Q. Examine the role of India's pharmaceutical industry in its soft power diplomacy. 150 words |
India is the largest supplier of generic drugs globally, providing over 20% of the world's generic medicines by volume.
A generic medicine is a copy of a branded drug with the same active ingredients, dosage, and quality, but sold at a much lower price after the original patent expires.
It's a provision under the Indian Patents Act and the TRIPS Agreement that allows the government to authorize a third party to produce a patented drug without the consent of the patent holder under specific conditions, primarily for public health emergencies.




© 2026 iasgyan. All right reserved