Description
Disclaimer: Copyright infringement not intended.
Context
The Department of Biotechnology (DBT) officially announced the completion of the ‘10,000 genome’ project — an attempt to create a reference database of whole-genome sequences out of India.
Details
- Population Diversity: India's vast population of 1.3 billion comprises over 4,600 distinct population groups, many of which are endogamous. This diversity contributes to unique genetic variations and disease-causing mutations within the Indian population.
- Institutions Involved: Approximately 20 institutions across India participated in the project, with the Indian Institute of Science (IISc), Bengaluru, and the Centre for Cellular and Molecular Biology (CCMB), Hyderabad, leading the initiative.
Implications
- Understanding Genetic Variants: The project aims to identify genetic variants unique to India's population groups, facilitating the customization of drugs and therapies tailored to individual genetic profiles.
- Medical Applications: By gaining deeper insights into India's population diversity and genetic predispositions to diseases, the project can improve diagnostic methods, medical counseling, and the development of personalized drugs.
- Future Research Directions: The creation of a biobank housing 20,000 blood samples, coupled with data archiving at the Indian Biological Data Centre, demonstrates the project's commitment to transparency, collaboration, and future research endeavors.
Genome India Project
- Launched in 2020, GIP draws inspiration from the success of the international Human Genome Project, which decoded the entire human genome between 1990 and 2003.
- Objectives:
- To comprehend the genetic variations and disease-causing mutations prevalent in the Indian population.
- To facilitate the development of personalized drugs and therapies tailored to the genetic makeup of individuals.
- Project Overview:
- Scope: GIP aims to sequence and analyze the genomes of 10,000 individuals by the end of 2023.
- Collaborative Effort: Involving 20 institutions across India, the project operates under the leadership of the Centre for Brain Research at the Indian Institute of Science, Bangalore.
- Current Progress: As of now, close to 7,000 genomes have been sequenced, with 3,000 already accessible to the public.
Applications
- Biotechnology: Genetic data can fuel advancements in biotechnology, enabling the development of novel treatments and technologies.
- Agriculture: Understanding genetic variations can aid in crop improvement and agricultural practices suited to diverse Indian environments.
- Healthcare: Personalized medicine and diagnostics based on genetic predispositions can revolutionize healthcare delivery, improving treatment efficacy and patient outcomes.

Human Genome Project
Objectives and Timeline
- Initiation: Launched in 1990, the HGP aimed to decode the entire euchromatic human genome sequence within 15 years.
- Collaboration: Coordinated by the National Institutes of Health (NIH) and the U.S. Department of Energy, the project involved an international consortium of scientists and researchers.
Scope and Achievements
- Genome Sequencing: The primary goal was to determine the sequence of the human genome, comprising approximately 3 billion nucleotide base pairs.
- Gene Identification: In addition to sequencing, the project aimed to identify and catalog all the genes present in the human genome.
- Technological Advancements: The HGP spurred significant innovations in DNA sequencing technologies, leading to faster, more accurate, and cost-effective methods.
Impact on Medicine and Technology
- Personalized Medicine: The wealth of genetic information generated by the HGP paved the way for personalized medicine, wherein treatments are tailored to an individual's genetic makeup.
- Drug Development: Insights from the HGP have facilitated the development of targeted therapies, such as Her2/neu for breast cancer and CYP450 testing for antidepressant response.
- Understanding Diseases: Genome-wide association studies (GWAS) made possible by the HGP have provided crucial insights into the genetic basis of various diseases, aiding in diagnosis, prevention, and treatment.
- Bioinformatics: The need to manage and analyze vast amounts of genomic data spurred advancements in bioinformatics and computational biology.
Introduction to Genomes
- Definition: A genome is the complete set of genetic material within an organism. It contains all the information needed for the development, functioning, and reproduction of that organism.
- Genetic Material: Genomes are composed of DNA (deoxyribonucleic acid) in most organisms, while some viruses may use RNA (ribonucleic acid) as their genetic material.
- Importance: Understanding genomes is crucial for unraveling the fundamental mechanisms of life, from evolution to disease.
Structure of Genomes
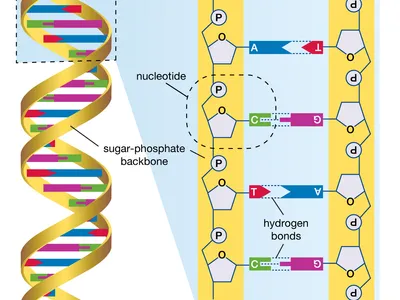
- DNA Molecules:
- DNA is composed of two long strands forming a double helix.
- Nucleotides: The building blocks of DNA, consisting of a sugar molecule (deoxyribose), a phosphate group, and one of four nitrogenous bases (adenine, thymine, cytosine, and guanine).
- Genes:
- Functional units of DNA responsible for encoding proteins or RNA molecules.
- Genes contain instructions for synthesizing specific molecules, such as enzymes or structural proteins.
- Chromosomes:
- DNA is organized into chromosomes, which are thread-like structures containing large DNA molecules and associated proteins.
- Humans have 46 chromosomes organized into 23 pairs, including one pair of sex chromosomes.
- Genome Size and Complexity:
- Genome sizes vary greatly among organisms, from a few thousand base pairs in some viruses to billions of base pairs in complex organisms like humans.
Functionality of Genomes
- Gene Expression:
- The process by which information from the genome is used to synthesize functional molecules (proteins or RNA).
- Involves transcription (DNA to RNA) and translation (RNA to protein).
- Regulation:
- Mechanisms that control when and where genes are expressed.
- Includes transcription factors, epigenetic modifications, and non-coding RNAs.
- Genetic Variation:
- Differences in DNA sequences among individuals or populations.
- Contributes to diversity, evolution, and susceptibility to diseases.
Genome Sequencing and Analysis
- Genome Sequencing Techniques:
- Sanger sequencing, Next-Generation Sequencing (NGS), and more recently, Third-Generation Sequencing (such as PacBio and Nanopore).
- Bioinformatics:
- Computational methods for analyzing and interpreting genomic data.
- Tasks include genome assembly, variant calling, and comparative genomics.
- Applications:
- Medical genetics, personalized medicine, evolutionary biology, agriculture, and forensic science.
Challenges and Ethical Considerations
- Data Privacy and Security:
- Concerns regarding the storage and use of personal genomic information.
- Genetic Discrimination:
- Risks of discrimination based on genetic predispositions or susceptibilities.
- Access and Equity:
- Ensuring equitable access to genomic technologies and benefits across diverse populations.
- Ethical Use of Genetic Information:
- Guidelines for responsible research and clinical practices.
Introduction to Genome Sequencing
- Definition: Genome sequencing is the process of determining the precise order of nucleotides within an organism's DNA.
- Historical Background:
- Milestones include the development of Sanger sequencing in the 1970s and the advent of Next-Generation Sequencing (NGS) technologies in the 2000s.
- Importance: Genome sequencing enables scientists to understand genetic variation, study evolutionary relationships, diagnose diseases, and develop personalized treatments.
Techniques of Genome Sequencing
- Sanger Sequencing:
- Developed by Fred Sanger in the 1970s.
- Involves DNA replication with chain-terminating dideoxynucleotides (ddNTPs) and gel electrophoresis to determine the sequence.
- Next-Generation Sequencing (NGS):
- High-throughput methods that parallelize sequencing reactions, enabling faster and cheaper sequencing.
- Techniques include Illumina sequencing, Ion Torrent sequencing, and Roche 454 sequencing.
- Third-Generation Sequencing:
- Overcomes limitations of NGS by sequencing single DNA molecules in real-time.
- Technologies include PacBio (Pacific Biosciences) and Oxford Nanopore sequencing.
Steps in Genome Sequencing
- Sample Preparation:
- Extraction of DNA from the organism of interest.
- Fragmentation of DNA into smaller pieces for sequencing.
- Library Preparation:
- Addition of adapters to DNA fragments to enable sequencing.
- Amplification of DNA fragments to increase sequencing signal.
- Sequencing:
- Performing the sequencing reaction using the chosen technology.
- Generating raw sequence data (reads).
- Data Analysis:
- Alignment of reads to a reference genome or de novo assembly.
- Variant calling to identify genetic variations.
- Interpretation:
- Annotation of genetic variants to understand their functional significance.
- Correlation of genetic findings with phenotypic traits or diseases.
Applications of Genome Sequencing
- Medical Genetics:
- Diagnosis of genetic disorders and predispositions.
- Pharmacogenomics for personalized medicine.
- Evolutionary Biology:
- Reconstruction of evolutionary relationships among species.
- Study of genetic adaptation and diversification.
- Agriculture:
- Improvement of crops and livestock through selective breeding.
- Identification of genetic markers for desirable traits.
- Microbiology:
- Characterization of microbial communities in environmental and clinical samples.
- Tracking the spread of infectious diseases.

Challenges
- Cost and Accessibility:
- Genome sequencing technologies have become more affordable but still require significant resources.
- Ensuring equitable access to sequencing technologies globally.
- Data Analysis and Interpretation:
- Handling and interpreting large volumes of sequencing data.
- Integrating genetic findings with clinical data for actionable insights.
- Ethical and Privacy Concerns:
- Protecting the privacy of individuals' genetic information.
- Addressing issues of consent and data ownership.
- Technological Limitations:
- Error rates and limitations in sequencing accuracy.
- Challenges in sequencing repetitive or complex regions of the genome.
Conclusion
The completion of the '10,000 genome' project marks a significant milestone in India's efforts to advance genetic research and personalized medicine. By creating a comprehensive database of whole-genome sequences, the project sets the stage for further breakthroughs in understanding genetic diversity, disease susceptibility, and tailored healthcare solutions for India's diverse population.
|
PRACTICE QUESTION
Q. Genome sequencing has revolutionized biological research and healthcare, providing unprecedented insights into the structure and function of genomes. Discuss. (250 words)
|











