



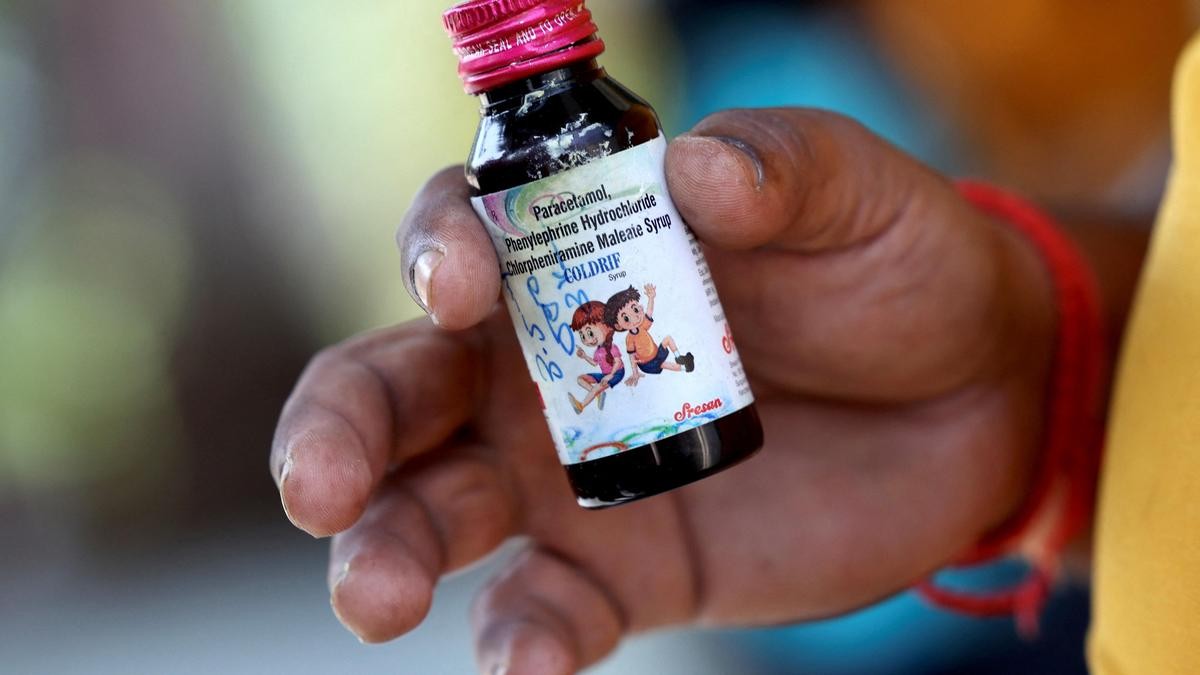
Recent child deaths in Madhya Pradesh and Rajasthan have been linked to contaminated cough syrups containing toxic industrial solvents. These substandard medicines pose serious health risks, especially to children. India struggles with weak drug regulation, outdated laws, and limited enforcement capacity.

Copyright infringement not intended
Picture Courtesy: Indian Express
Recent deaths of children in Madhya Pradesh and Rajasthan linked to contaminated cough syrups have caused widespread concern among parents.
Source: Indian Express and The BMJ
|
Practice Question Q. Examine the key challenges in eradicating counterfeit and substandard medicines from the Indian pharmaceutical system and suggest a multi-pronged strategy to address these challenges, focusing on regulatory reform, technology adoption, and public awareness. |
Contaminated medicine refers to drugs that contain harmful substances or have been compromised during manufacturing, packaging, or distribution.
Common causes include:





© 2026 iasgyan. All right reserved