
Disclaimer: Copyright infringement not intended.
Context
- John B Goodenough died on June 25 at the age of 100.
John B Goodenough
About
- John Bannister Goodenough was an American materials scientist, a solid-state physicist, and a Nobel laureate in chemistry.
Contribution
- He is widely credited with:
- the identification and development of the lithium-ion battery,
- for developing the Goodenough–Kanamori rules in determining the sign of the magnetic superexchange in materials, and
- for seminal developments in computer random-access memory (RAM)
Fundamental Investigations
- Goodenough’s research focused on magnetism and on the Metal–insulator transition behavior in transition-metal oxides.
- Along with Junjiro Kanamori, Goodenough developed a set of semi-empirical rules to predict magnetism in these materials in the 1950s and 1960s, now called the Goodenough–Kanamori rules, forming the basis of superexchange, which is a core property for high-temperature superconductivity.
Recognition
- Goodenough was awarded the Nobel Prize in Chemistry on October 9, 2019, for his work on lithium-ion batteries.

Trivia: The journey of the development of Lithium-Ion Batteries
First True Battery
- The first true battery was created in 1800 by Alessandro Volta.
- Using disks of copper and zinc, linked with a cloth soaked in salty water, he was able to generate electricity.
First Rechargeable Battery
- One of the first rechargeable batteries was invented some six decades later — they were lead-acid batteries, prominently used to power car ignition and its accessories like lights.
- There were several issues with them such as their short life cycle, bulk, high maintenance, and slow and inefficient charging.
First lithium-based rechargeable battery
- A breakthrough came in the early 1970s when Whittingham developed the first lithium-based rechargeable battery.
- He used lithium for his battery’s “negative electrode (anode), and titanium disulfide (disulphide), not previously used in batteries, for its positive electrode (cathode).”
- The result was a groundbreaking invention as Whittingham’s innovative battery could produce high voltage, work at room temperature and was rechargeable.
- He consciously used lithium because it easily releases electrons to travel to the cathode, making the battery work.
- However, Whittingham’s batteries had some shortcomings — they would either explode or catch fire in case of overcharging or repeated recharging. This is where Goodenough came into the picture.
Contribution of Goodenough in lithium-based rechargeable battery
- Being aware of Whittingham’s invention, Goodenough realised that if he made the battery’s cathode from a metal oxide and lithium instead of a metal disulphide, it might have “higher potential”.
- The metal oxide that he and his team finally zeroed in on was cobalt oxide.
- Whittingham’s battery generated more than two volts, but Goodenough discovered that the battery with lithium cobalt oxide in the cathode was almost twice as powerful, at four volts.
- The original lithium-cobalt-oxide cathode structure of Goodenough is still used in the lithium-ion batteries that today power most of the gadgets.
First commercially viable lithium-ion battery
- Yoshino, who had been experimenting with the battery by using carbon-based materials as its anode.
- He ultimately made a breakthrough when he used petroleum coke as an anode, which, at the molecular level, has spaces for lithium ions.
- When he charged the petroleum coke with electrons, the lithium ions were drawn into the material. Then, when he turned on the battery, the electrons and lithium ions flowed towards the cobalt oxide in the cathode, which has a much higher potential.
- This way, Yoshino invented the first commercially viable lithium-ion battery, which began to be sold in 1991. It was powerful, lightweight, stable and offered a long life.
Mainstreaming of Lithium-Ion Batteries
- Lithium-ion batteries became mainstream when Sony started to sell them, sparking a revolution in the electronics industry.
- It finally paved the way for the emergence of mobile phones and laptops.
- Not only this, lithium-ion batteries are a crucial part of electrical vehicles, which are pivotal to the world’s transition to clean energy.
READ ABOUT LITHIUM: https://www.iasgyan.in/daily-current-affairs/lithium
READ ABOUT LITHIUM ION BATTERY: https://www.iasgyan.in/daily-current-affairs/lithium-ion-battery

Lithium-Ion Battery
History
- The idea of Lithium Ion battery was first coined by G.N Lewis in 1912, but it became feasible only in the year 1970’s and the first non-rechargeable lithium battery was put into commercial markets.
What is a lithium-ion battery?
- A lithium-ion battery is a type of rechargeable battery that is charged and discharged by lithium ions moving between the negative (anode) and positive (cathode) electrodes.
Generally, batteries that can be charged and discharged repeatedly are called secondary batteries, whereas disposable batteries are called primary batteries.
- Because lithium-ion batteries are suitable for storing high-capacity power, they are used in a wide range of applications, including consumer electronics such as smartphones and PCs, industrial robots, production equipment and automobiles.
What are the components of a lithium-ion cell?
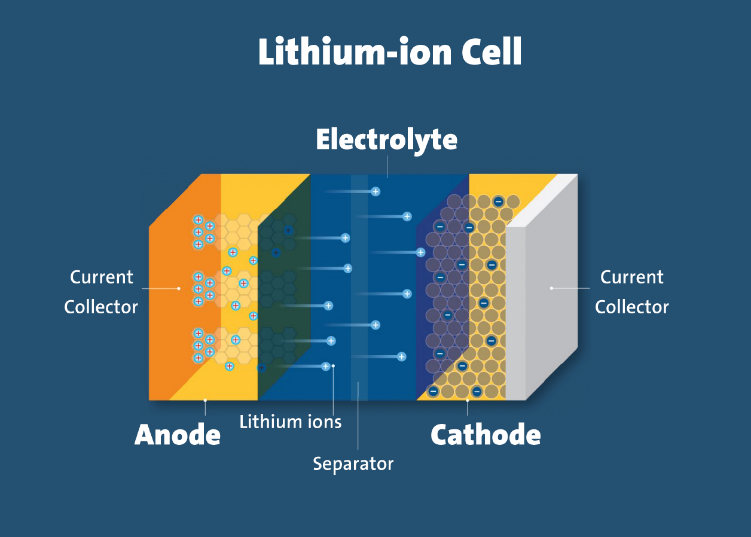
- Electrodes: The positively and negatively charged ends of a cell. Attached to the current collectors
- Anode: The negative electrode
- Cathode: The positive electrode
- Electrolyte: A liquid or gel that conducts electricity
- Current collectors: Conductive foils at each electrode of the battery that are connected to the terminals of the cell. The cell terminals transmit the electric current between the battery, the device and the energy source that powers the battery
- Separator: A porous polymeric film that separates the electrodes while enabling the exchange of lithium ions from one side to the other
How does a lithium-ion cell work?
- In a lithium-ion battery, lithium ions (Li+) move between the cathode and anode internally. Electrons move in the opposite direction in the external circuit. This migration is the reason the battery powers the device—because it creates the electrical current.
- While the battery is discharging, the anode releases lithium ions to the cathode, generating a flow of electrons that helps to power the relevant device.
- When the battery is charging, the opposite occurs: lithium ions are released by the cathode and received by the anode.
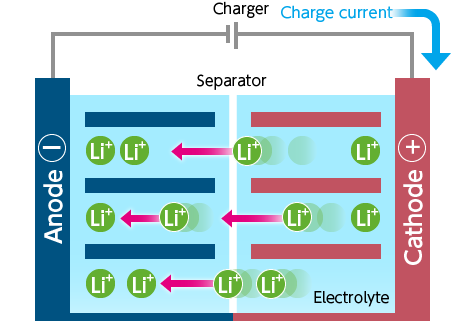
- The charger passes current to the battery.
- Lithium ions move from the cathode to the anode through the electrolyte.
- The battery is charged by a potential difference between the two electrodes.
When using energy (i.e., during discharging)
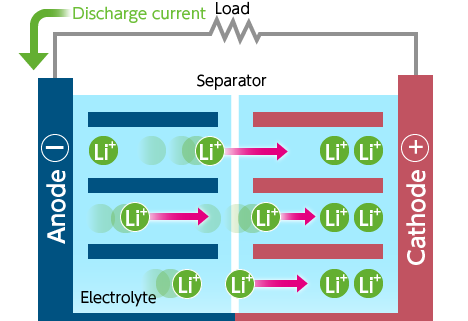
- A discharge circuit is formed between the anode and the cathode.
- Lithium ions stored in the anode move to the cathode.
- Energy is used.
The battery can be charged and discharged many times through the movement of lithium ions.
How do lithium-ion batteries compare with lead-acid ones?
- Generally, lithium-ion batteries are lighter and can be charged more rapidly than lead-acid batteries.
- And lithium-ion batteries are more environmentally friendly since they don't contain any substance with a high environmental load.
FAST FACTS
- Much cleaner than other kinds, Lithium Ion Battery is safer for the environment and can be recycled.
- Li-ion batteries are capable of high voltage and charge storage per unit mass and volume.
- Li-ion batteries can use different materials as electrodes — lithium cobalt oxide (cathode) and graphite (anode) is the most common combination in portable devices such as mobile phones and laptops.
- Lithium-ion batteries weigh one-third of lead-acid batteries. Lithium-ion batteries are nearly 100 per cent efficient in both charge and discharge, allowing for the same amp hours in and out, unlike the amp and voltage loss in lead-acid batteries.
- Li-ion must be sent to certified battery recyclers and not discarded in a trash bin.
- Despite the higher upfront cost, compared with lead-acid, the true cost of lithium-ion batteries is far less due to their longer life span and performance.
|
PRACTICE QUESTION
Q. Consider the following statements with respect to Lithium Ion Batteries:
1. The idea of the Lithium-Ion battery was first coined by G.N Lewis in 1912.
2. Lithium-ion batteries weigh one-third of lead-acid batteries.
3. Li-ion batteries cannot use different materials as electrodes.
4. Li-ion batteries are capable of high voltage and charge storage per unit mass and volume.
Which of the above statements is/are incorrect?
(a) 1 and 4 only
(b) 2, and 3 only
(c) 3 only
(d) None of the above.
Correct Answer: (c) 3 only
|
https://indianexpress.com/article/explained/explained-sci-tech/john-goodenough-dead-lithium-ion-battery-8689035/

















