




Copyright infringement not intended
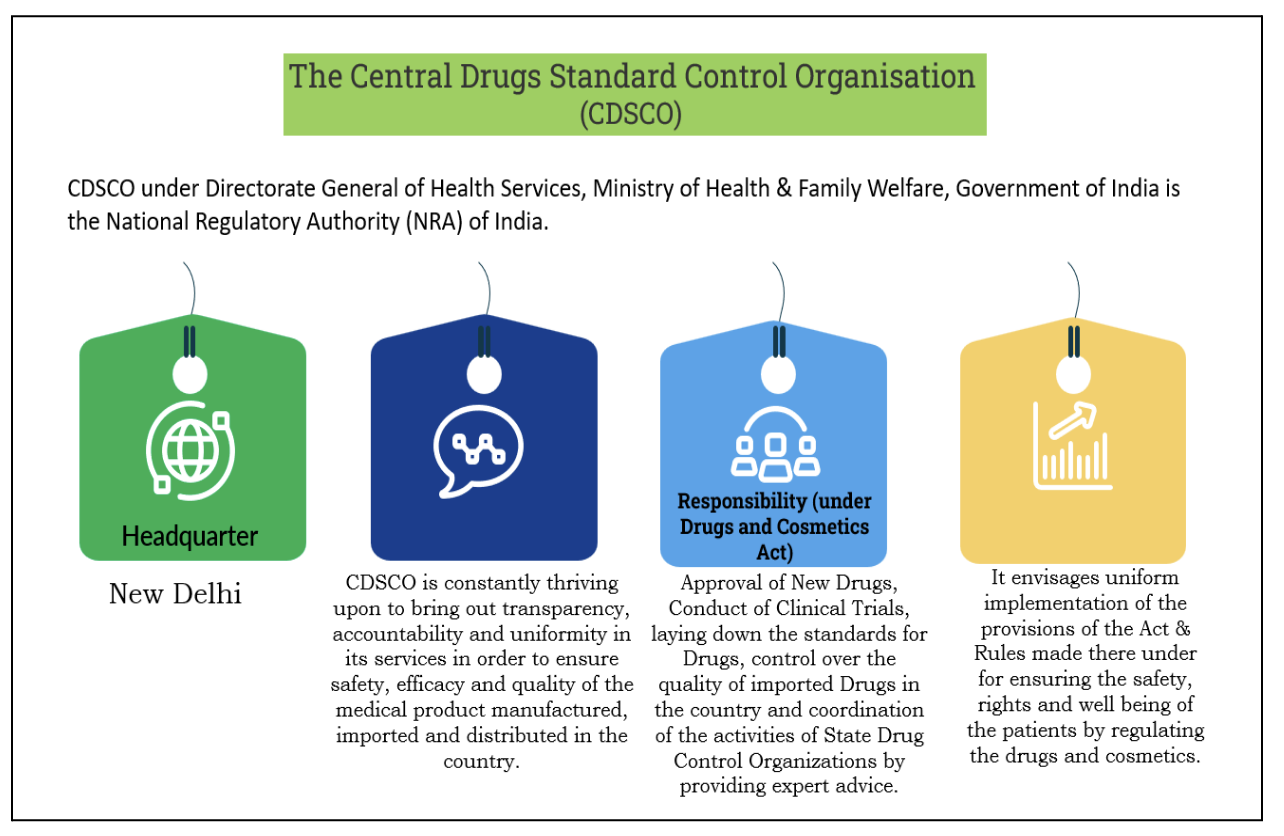
Context: The Union Government announced to centralize the authority for issuing manufacturing licenses and No Objection Certificates (NOCs) for certain categories of drugs meant for export.
Details
Key Changes and Implications
Significance
|
Recent Data released by the Commerce Ministry ●India's pharmaceutical exports increased by 9.67% year-on-year to reach USD 27.9 billion in FY24, marking a significant increase from USD 25.4 billion recorded in the previous fiscal year. ●The United States, the United Kingdom, the Netherlands, South Africa, and Brazil emerged as the top five export destinations for Indian pharmaceutical products during FY24. The US alone accounted for over 31% of India's total pharma exports. ●India's pharmaceutical industry is recognized as the third-largest by volume and the 13th-largest by value globally. It has a vast portfolio of more than 60,000 generic drugs across 60 therapeutic categories, highlighting the sector's breadth and depth. ●The government has introduced two production-linked incentive (PLI) schemes aimed at promoting domestic manufacturing of key pharmaceutical ingredients and generic medicines, highlighting its commitment to promoting the growth and competitiveness of the sector. ●Despite global economic challenges, India's pharmaceutical sector has demonstrated resilience and driven significant export growth in FY24. With a strategic focus on diversification, innovation, and domestic manufacturing, the industry is well-positioned to capitalize on emerging market opportunities and sustain its growth in the coming years. |
Conclusion
Source:
|
PRACTICE QUESTION Q. India is known as the "Pharmacy of the World" for its dominance in generic drugs. How can Indian pharmaceutical companies move beyond generics and establish themselves as leaders in innovative drug discovery, considering the challenges of high R&D costs, stricter regulations for new drugs, and competition from established multinational corporations? |





© 2026 iasgyan. All right reserved